While this law specifically applies to ideal gases, most gases approximate the ideal gas law under most conditions. Ideal gas laws are used to find the species partial pressures and hence cathode exit pressure the ideal gas laws work well at relatively low pressures and relatively high temperatures. The ideal gas law is the equation of state for a hypothetical gas. It's very simple, easy to use, and easy to understand. So when we talk about elastic collisions, we are taking the kinetic energy as conserved and then finding appropriate values of velocities that would allow the kinetic energy to be conserved and simultaneously obey the law of.
Assuming that we understand the ideal gas law and the pvt relationship between pressure, volume, and temperature, it is a lot easier to remember just. What follows is just one way to derive the ideal gas law. So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time. Say out loud liter atmospheres per mole kelvin. this is not the only value of r that can exist.

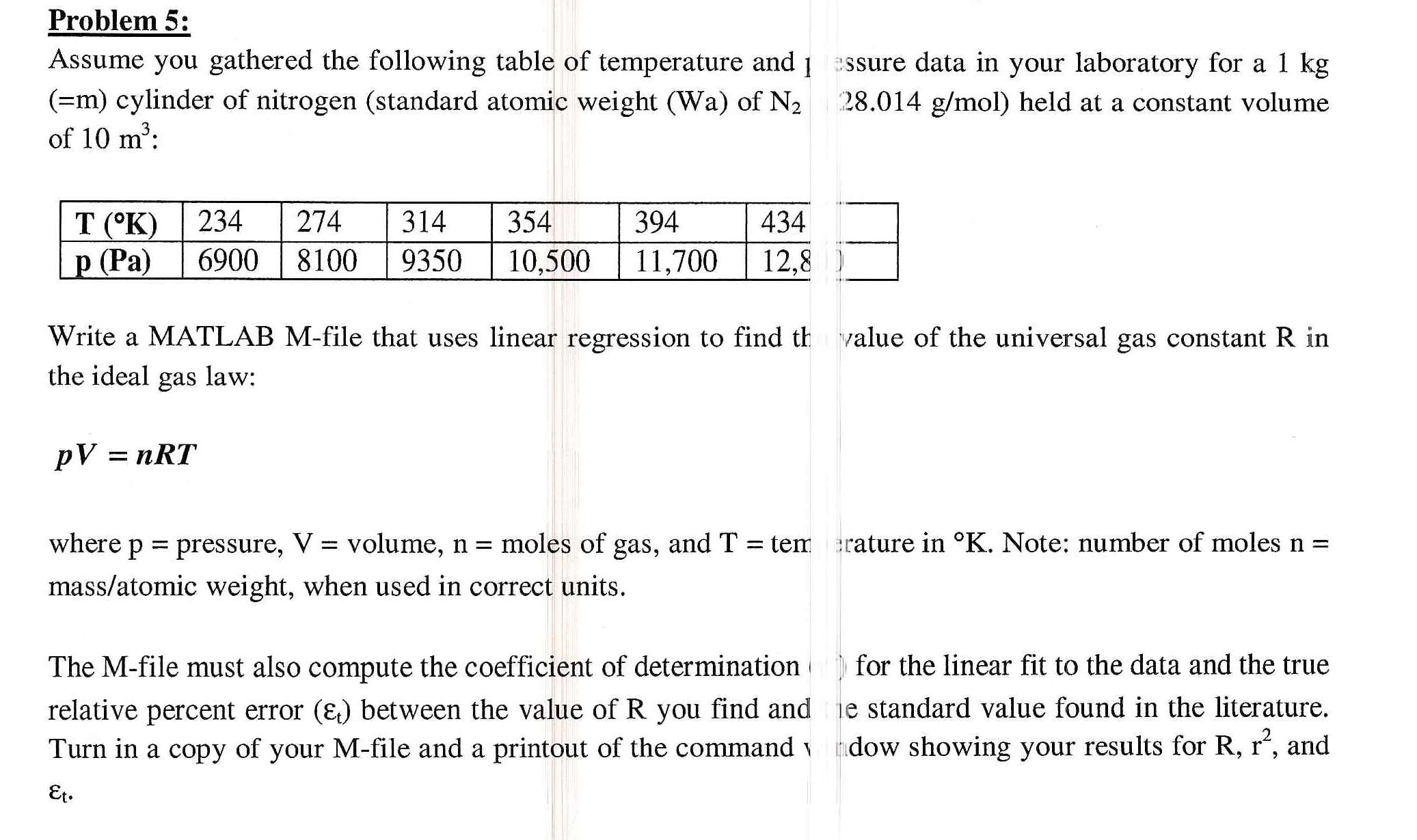
Ideal gas law calculations pv=nrt tutorial with worked examples for chemistry students.
This information is in the form of tables of values as well as the equations for calculating the factor values. Due to this fact the ideal gas law will only give an approximate value for real gases under normal condition that are not currently approaching qualification. The constant r is called the ideal gas law constant. The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures. One modified form of the ideal gas equation is to involve the density (d) and molecular weight (m) instead of volume (v) and. The ideal gas law is the equation of state of an ideal gas (also known as a perfect gas) that relates its absolute pressure p to its absolute temperature t. Ideal gas law or perfect gas law represents the mixed relationship between pressure, volume, the temperature of gases for learning the ideal gas equation balancing these state variables in terms of universal gas constant (r). The three historically important gas laws derived relationships between two physical properties of a rearranging to a more familiar form: Real gases are dealt with in more detail on another page. Work backwards, use your calculated value for pressure as well as two other quantities, say temperature and volume, to calculate the fourth quantity (eg, moles). The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time. The classical carnot heat engine. The ideal gas law is the equation of state for a hypothetical gas.
The value and units of r depend on the units used in determining p, v. One modified form of the ideal gas equation is to involve the density (d) and molecular weight (m) instead of volume (v) and. Below are 47 working coupons for values of r in ideal gas law from reliable websites that we have updated for users to get maximum savings. Its value depends on the units used. A gas whose particles exhibit no attractive interactions whatsoever; Apply the ideal gas law to molar volumes, density, and stoichiometry problems. Further parameters that enter the equation are the volume v of the container holding the gas and the amount n (in moles) of gas contained in there. Ideal gas law calculations pv=nrt tutorial with worked examples for chemistry students.
Further parameters that enter the equation are the volume v of the container holding the gas and the amount n (in moles) of gas contained in there.
It is the molar equivalent to the boltzmann constant, expressed in units of energy per temperature increment per mole, i.e. If the pressure p is in atmospheres (atm), the volume v is in liters (l), the moles n is in moles (mol), and temperature t is in kelvin (k), then r lastly, this video may help introduce you to the ideal gas law. It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value. The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. Here are the steps to follow when using this online tool The kinetic theory of gases. The ideal or perfect gas law formula can use for calculating the value of. The ideal gas law is the equation of state of a hypothetical ideal gas. So when we talk about elastic collisions, we are taking the kinetic energy as conserved and then finding appropriate values of velocities that would allow the kinetic energy to be conserved and simultaneously obey the law of. This ideal gas law calculator is also known as a gas pressure calculator, a molar volume calculator or a gas volume calculator because you can use it to find different values. Due to this fact the ideal gas law will only give an approximate value for real gases under normal condition that are not currently approaching qualification.
Value of r will change when dealing with different unit of pressure and volume (temperature factor is overlooked because temperature will always be in kelvin instead of celsius when using the ideal gas. Say out loud liter atmospheres per mole kelvin. this is not the only value of r that can exist. Notice the weird unit on r: To find any of these values, simply enter the other ones into the ideal gas law calculator. Values of r in the perfect gas law here comes the tricky part when it comes to the gas constant, r. The ideal gas law states that p x v = n x r x t where, p is pressure, v is volume, n is number of moles of the gas, r is the ideal gas constant and t is temperature in kelvin. The value and units of r depend on the units used in determining p, v. Apply the ideal gas law to molar volumes, density, and stoichiometry problems. The approximate value is generally accurate under many conditions.
The constant r is called the ideal gas law constant.
At high ideal gas law introduction: It is a combination of the previous laws that we have studied (boyle's, charles', avogadro's). You'll need it for problem solving. What follows is just one way to derive the ideal gas law. The ideal gas law is the equation of state for a hypothetical gas. Further parameters that enter the equation are the volume v of the container holding the gas and the amount n (in moles) of gas contained in there. It is the molar equivalent to the boltzmann constant, expressed in units of energy per temperature increment per mole, i.e. This ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. The constant r is called the gas constant. The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures. The ideal gas law was first written in 1834 by emil clapeyron.

Say out loud liter atmospheres per mole kelvin. this is not the only value of r that can exist.

If the pressure p is in atmospheres (atm), the volume v is in liters (l), the moles n is in moles (mol), and temperature t is in kelvin (k), then r lastly, this video may help introduce you to the ideal gas law.

The ideal gas law is the equation of state of a hypothetical ideal gas.

Notice the weird unit on r:

Assuming that we understand the ideal gas law and the pvt relationship between pressure, volume, and temperature, it is a lot easier to remember just.

The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.

What follows is just one way to derive the ideal gas law.
Tt of an ideal gas are related by a simple formula called the ideal gas law.

At high ideal gas law introduction:

To find any of these values, simply enter the other ones into the ideal gas law calculator.

What follows is just one way to derive the ideal gas law.
.PNG)
Instead of using the regular ideal gas equation.

There is no such thing as an ideal gas, of course, but many gases behave approximately as if they were ideal at ordinary working temperatures and pressures.

One modified form of the ideal gas equation is to involve the density (d) and molecular weight (m) instead of volume (v) and.

The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures.

Due to this fact the ideal gas law will only give an approximate value for real gases under normal condition that are not currently approaching qualification.
Substituting the values for the number of moles, the appropriate ideal gas constant, the absolute temperature, and the absolute pressure gives.

Notice the weird unit on r:

Apply the ideal gas law to molar volumes, density, and stoichiometry problems.

Value of r will change when dealing with different unit of pressure and volume (temperature factor is overlooked because temperature will always be in kelvin instead of celsius when using the ideal gas.

Values of r (gas constant).

The ideal gas law is a simple model that allows us to predict the behavior of gases in the world.

The classical carnot heat engine.

What follows is just one way to derive the ideal gas law.
If the pressure p is in atmospheres (atm), the volume v is in liters (l), the moles n is in moles (mol), and temperature t is in kelvin (k), then r lastly, this video may help introduce you to the ideal gas law.

Instead of using the regular ideal gas equation.

At high ideal gas law introduction:

Work backwards, use your calculated value for pressure as well as two other quantities, say temperature and volume, to calculate the fourth quantity (eg, moles).

Lower pressure is best because then the average.

At high ideal gas law introduction:
Posting Komentar untuk "Ideal Gas Law R Values - Quia - Chap 11 Gases"